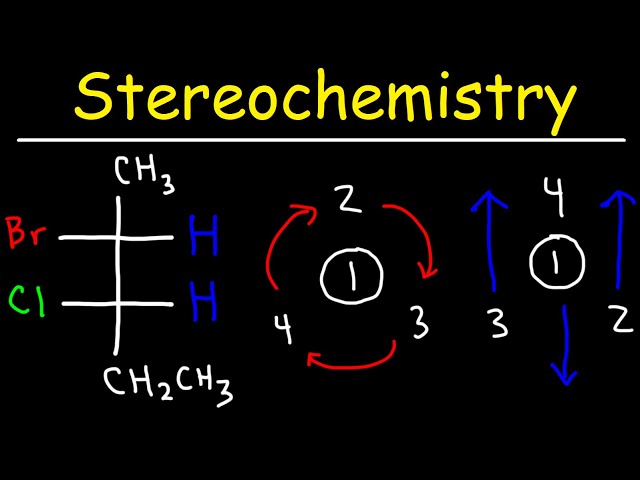
In stereochemistry, the term optically inactive refers to molecules that do not rotate plane-polarized light. Fisher projections are a way of representing molecules in two dimensions, and they can be used to determine whether a molecule is optically active or inactive. Examples of optically inactive Fisher projections include meso compounds, which have an internal plane of symmetry, and racemic mixtures, which are composed of equal amounts of two enantiomers.
To determine whether a molecule is optically inactive using a Fischer projection, you can follow these steps:
- Draw the Fischer projection of the molecule.
- Identify the chiral centers in the molecule. A chiral center is a carbon atom that is bonded to four different groups.
- Determine whether the molecule has an internal plane of symmetry. If the molecule has an internal plane of symmetry, then it is meso and optically inactive.
- If the molecule does not have an internal plane of symmetry, then determine whether the molecule is a racemic mixture. A racemic mixture is composed of equal amounts of two enantiomers, which are molecules that are mirror images of each other. Racemic mixtures are optically inactive.
Tips for Determining Optical Inactivity Using Fisher Projections
Here are a few tips for determining optical inactivity using Fisher projections:
- Tip 1: Look for molecules with an internal plane of symmetry. If a molecule has an internal plane of symmetry, then it is meso and optically inactive.
- Tip 2: Consider the possibility of racemization. If a molecule is a racemic mixture, then it is optically inactive. Racemization can occur when a chiral molecule is heated or exposed to certain chemicals.
- Tip 3: Use reference materials. There are a number of reference materials available that can help you to identify optically inactive molecules. These materials include tables of meso compounds and racemic mixtures.
Frequently Asked Questions About Optically Inactive Fisher Projections
Here are a few frequently asked questions about optically inactive Fisher projections:
- Question: What is the difference between a meso compound and a racemic mixture?
Answer: A meso compound is a molecule that has an internal plane of symmetry. A racemic mixture is a mixture of two enantiomers in equal amounts. Both meso compounds and racemic mixtures are optically inactive.
Question: How can I tell if a molecule is optically inactive by looking at its Fisher projection?
Answer: To tell if a molecule is optically inactive by looking at its Fisher projection, you can look for molecules with an internal plane of symmetry. If a molecule has an internal plane of symmetry, then it is meso and optically inactive. You can also consider the possibility of racemization. If a molecule is a racemic mixture, then it is optically inactive.
Optically inactive Fisher projections are an important tool for understanding the stereochemistry of molecules. They can be used to determine whether a molecule is optically active or inactive, and they can also be used to identify meso compounds and racemic mixtures.
Youtube Video: