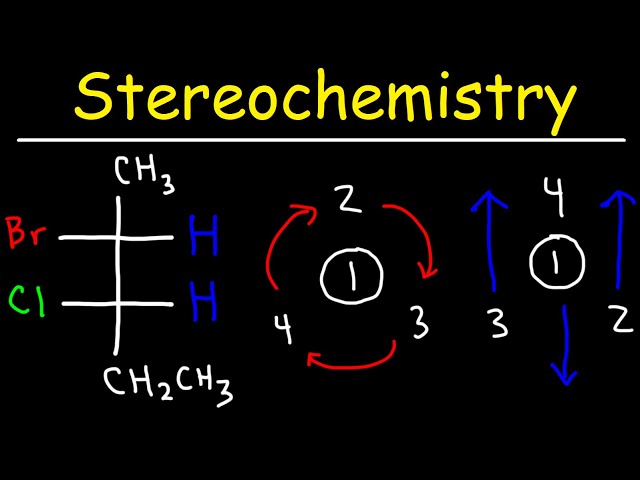
Examples of optically inactive fischer projection are crucial for a variety of reasons. In chemistry, Fischer projections are a way of representing three-dimensional molecules in two dimensions, analogous to how a blueprint represents a three-dimensional building. They are particularly useful for representing organic molecules, such as sugars and amino acids, which often have multiple chiral centers. A chiral center is a carbon atom that is bonded to four different groups, and it can exist in two different configurations, known as enantiomers. Enantiomers are mirror images of each other, and they have the same physical properties, such as melting point and boiling point. However, they can have different biological properties, such as the ability to interact with enzymes. Optically inactive Fischer projections are those that represent molecules that are not chiral. This means that they do not have any chiral centers, or that they have an equal number of chiral centers with opposite configurations.
One example of an optically inactive Fischer projection is the projection of methane. Methane has a carbon atom that is bonded to four hydrogen atoms, and all of the hydrogen atoms are equivalent. This means that methane does not have any chiral centers, and it is optically inactive. Another example of an optically inactive Fischer projection is the projection of ethane. Ethane has two carbon atoms that are bonded to each other, and each carbon atom is bonded to three hydrogen atoms. Again, all of the hydrogen atoms are equivalent, so ethane does not have any chiral centers and it is optically inactive.
Tips for drawing optically inactive Fischer projections
There are a few tips that can help you to draw optically inactive Fischer projections. First, it is important to remember that the Fischer projection is a two-dimensional representation of a three-dimensional molecule. This means that you need to be careful to represent the correct spatial relationships between the atoms. Second, it is important to use the correct line notation. In a Fischer projection, the horizontal lines represent bonds that are coming out of the plane of the paper, and the vertical lines represent bonds that are going into the plane of the paper. Finally, it is important to make sure that the Fischer projection is not chiral. This means that it should not have any chiral centers, or that it should have an equal number of chiral centers with opposite configurations.
1. 1. Identify the chiral centers in the molecule.
The first step in drawing an optically inactive Fischer projection is to identify the chiral centers in the molecule. A chiral center is a carbon atom that is bonded to four different groups. If a molecule has more than one chiral center, it is possible that it will be optically active.
2. 2. Assign priorities to the groups attached to each chiral center.
Once you have identified the chiral centers in the molecule, you need to assign priorities to the groups that are attached to each chiral center. The Cahn-Ingold-Prelog (CIP) priority rules are used to assign priorities to groups. The CIP priority rules are based on the atomic number of the atoms that are directly attached to the chiral center. The atom with the highest atomic number gets the highest priority. If two atoms have the same atomic number, then the atom that is bonded to the most other atoms gets the highest priority.
3. 3. Orient the molecule so that the lowest priority group is pointing away from you.
Once you have assigned priorities to the groups that are attached to each chiral center, you need to orient the molecule so that the lowest priority group is pointing away from you. This is known as the “highest priority rule.”
4. 4. Draw the Fischer projection.
Once you have oriented the molecule correctly, you can draw the Fischer projection. The Fischer projection is a two-dimensional representation of the molecule, and it shows the relative positions of the atoms and groups. The horizontal lines in the Fischer projection represent bonds that are coming out of the plane of the paper, and the vertical lines represent bonds that are going into the plane of the paper.
Frequently asked questions about optically inactive fischer projection
Here are some frequently asked questions about optically inactive Fischer projections:
Q: What is an optically inactive Fischer projection?
A: An optically inactive Fischer projection is a Fischer projection that represents a molecule that is not chiral. This means that the molecule does not have any chiral centers, or that it has an equal number of chiral centers with opposite configurations.
Q: How do you draw an optically inactive Fischer projection?
A: To draw an optically inactive Fischer projection, you need to follow these steps:
- Identify the chiral centers in the molecule.
- Assign priorities to the groups attached to each chiral center.
- Orient the molecule so that the lowest priority group is pointing away from you.
- Draw the Fischer projection.
Q: What are some examples of optically inactive Fischer projections?
A: Some examples of optically inactive Fischer projections include the projections of methane, ethane, and propane.
Conclusion
Optically inactive Fischer projections are an important tool for representing molecules that are not chiral. They are used in a variety of applications, such as chemistry, biochemistry, and pharmacology. By understanding how to draw and interpret optically inactive Fischer projections, you can gain a better understanding of the structure and properties of molecules.
Youtube Video: